- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us

Resolution ctDx Lung™
Provides clinicians with information that can drive treatment decisions in patients with NSCLC.
Order Resolution ctDx Lung Assay
If you have a Labcorp Oncology account, complete the test requisition form
If you do NOT have a Labcorp Oncology account, contact us for account setup
The Resolution ctDx LungTM assay includes actionable genes for targeted FDA-approved therapies or therapies in clinical trials.
Benefits
- An assay that only focuses on genes implicated in lung cancer
- Liquid biopsies can offer a complete picture of tumor heterogeneity
- Useful when tissue biopsies are limited or unobtainable from the patient
- Non-invasive method with testing performed on a blood sample
- Faster turn-around time compared to tissue NGS profiling1
Clinical Response Data
In a prospective clinical study, the Resolution ctDx Lung assay demonstrated the following performance1:
- Somatic mutations detected in 64% (135/210) of patients
97% (34/35) of patients who received plasma-directed therapy had a clinical and radiological response to the matched targeted therapy
46% (96/210) of patients had an oncogenic driver alteration detected, including actionable mutations in EGFR, ALK, MET, BRAF, ROS1, and RET
90% (60/67) positive concordance between plasma and tissue NGS testing. Sub-analysis demonstrated
96% (49/51) positive concordance within NCCN® oncogenic recognized driver alterations in lung cancer.
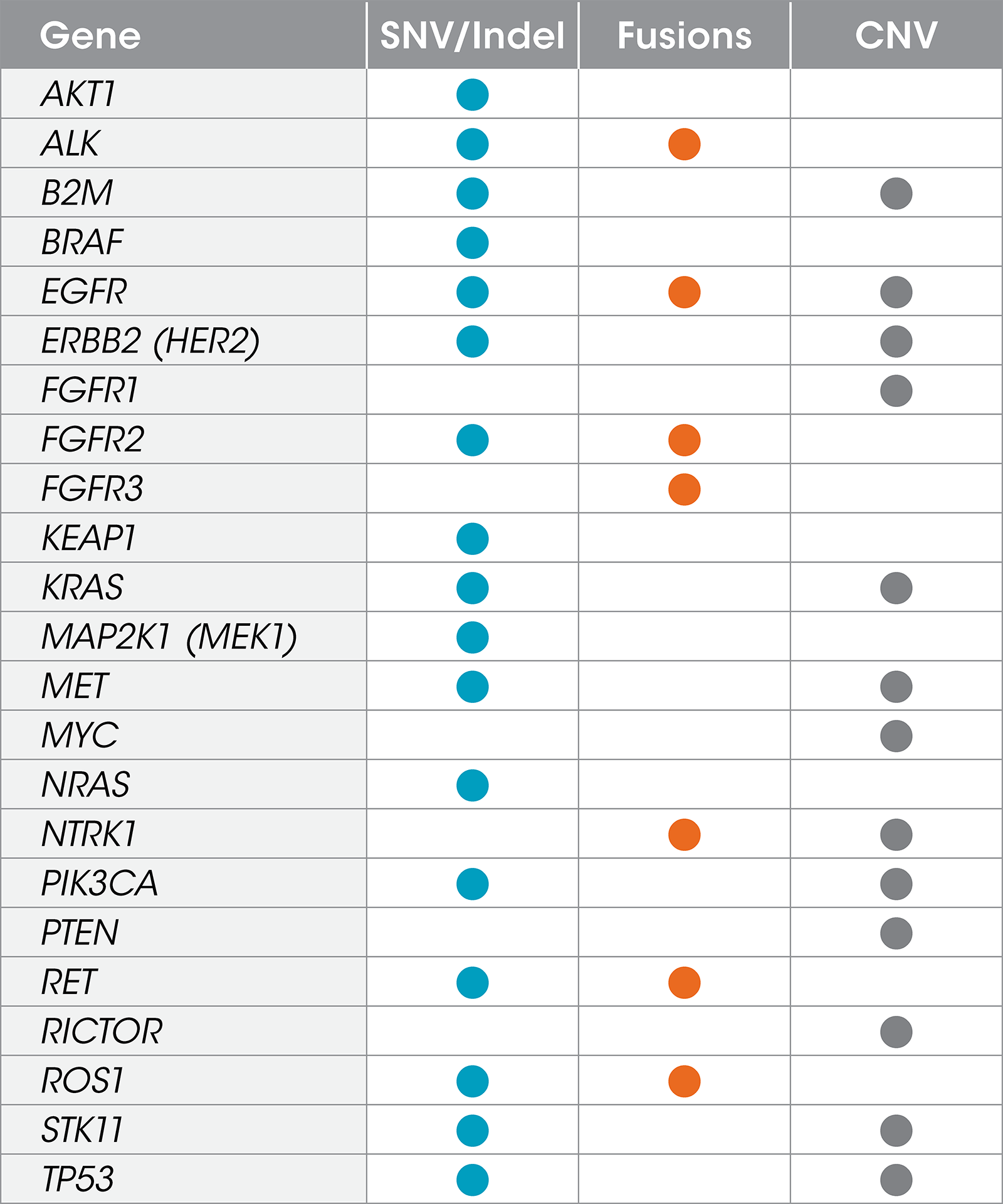
Gene List
The Resolution ctDx Lung™ assay targets actionable, somatic SNVs, indels,
fusions, and copy number variants in 22 genes in NSCLC.2
Please see the test menu for additional specimen and test information.
References
- Sabari, J. et al. A Prospective Study of Circulating Tumor DNA to Guide Matched Targeted Therapy in Lung Cancers; JNCI J Natl Cancer Inst 2019 111(6) djy156.
- Resolution Bioscience, Inc. ctDx Lung Panel. http://www.resolutionbio.com/assays/nsclc.html. Accessed March 9, 2020.



